Abstract
Background: MRD is an established biomarker to evaluate treatment efficacy, define patients at risk based on persistent MRD, and eventually, act as surrogate for prolonged survival based on sensitive MRD-negative definitions. Accordingly, the IMWG has developed criteria for MRD-negativity defined by next-generation sequencing, NGF or PET/CT, and has recommended their inclusion in clinical trials. Notwithstanding, most flow cytometry results have been obtained using less sensitive methods and in fact, there is no data about the impact of NGF-based MRD assessment in clinical trials.
Aim: To define the feasibility, sensitivity and clinical impact of NGF-based MRD assessment in the phase III PETHEMA/GEM2012 trial.
Methods: A total of 458 patients were enrolled into the PETHEMA/GEM2012 trial. MRD was predefined to be prospectively assessed at three time-points: after six induction cycles with bortezomib, lenalidomide, and dexamethasone (VRD), after HDT/ASCT, and after two courses of consolidation with VRD. MRD monitoring was performed blinded for clinical outcomes in four PETHEMA/GEM laboratory cores, and data was centralized for MRD analyses. MRD assessment was performed following EuroFlow SOPs in a total of 1,134 bone marrow (BM) samples from 419 patients. The 39 cases without MRD assessment had suboptimal response to induction and were thus considered as MRD+ for intention-to-treat analyses. Noteworthy, in 14 BM samples with undetectable MRD, B-cell precursors, erythroblasts and mast cells represented <0.01% of BM cells, and these samples were thus considered as hemodiluted and inadequate for MRD assessment. The limit of detection (LOD) was determined for each of the 1,117 BM samples representative for MRD assessment, according to the formula: (20/nucleated viable cells) x 100; the median LOD achieved by NGF in the PETHEMA/GEM2012 trial was of 3x10-6.
Results: Overall, 225/458 (49%) patients had undetectable MRD at the latest time-point in which MRD was assessed and were thus classified as MRD-. Conversely, 233/458 (51%) cases remained MRD+: 28% with ≥10-4 MRD, 12% with 10-5 MRD, and 11% with 10-6 MRD. Detailed analyses of MRD kinetics in 320 patients with available MRD results at all three time-points, showed that the percentage of MRD- patients increased from 35% into 54% and 58% after induction, HDT/ASCT and consolidation, respectively. Furthermore, a restricted analysis among MRD+ patients showed that whereas after induction only 8% of them had MRD levels as low as 10-6, subsequent intensification with HDT/ASCT and consolidation could reduce MRD levels down to 10-6 in 32% of MRD+ cases.
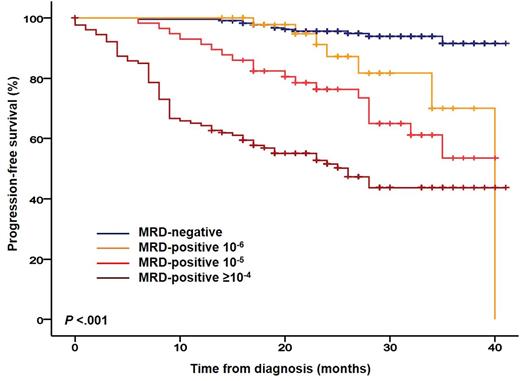
Progression-free survival (PFS) rates at 3-years were of 92%, 70%, 54% and 44% for patients being MRD-negative, MRD+ 10-6, 10-5 and ≥10-4, respectively (P<.001; Figure). Thus far, only 6/225 (3%) MRD- patients have relapsed; strikingly, all 6 cases had extramedullary plasmacytomas at diagnosis, all relapsed with extramedullary plasmacytomas, and only 2 had concomitant serological relapse. The favorable outcome of MRD- patients encouraged us to investigate the impact of MRD negativity in both standard- and high-risk patients defined by FISH [i.e.: t(4;14), t(14;16), and/or del(17p)]. Even though MRD- rates were significantly inferior in patients with high- vs standard-risk FISH (37% vs 50%, respectively; P=.03), 3-year PFS rates were similar between patients with high- and standard-risk FISH reaching MRD-negativity (94% and 91%, respectively; P=.56); by contrast, MRD+ cases with high- and standard- risk FISH had median PFS of 27 and 35 months, respectively (P=.025).
Conclusions: This is the largest study of MRD monitoring in MM based on the total number of samples analyzed (n=1,134). Our results show that NGF-based MRD assessment is feasible in large multicenter clinical trials, is highly-sensitive, and allows the identification of hemodiluted BM samples inadequate for MRD assessment. Risk of relapse among MRD-negative patients was remarkably reduced (3%), and was particularly related to the reappearance of extramedullary plasmacytomas, which urges the need for combined cellular and imaging MRD monitoring in these patients; by contrast, even MRD levels as low as 10-5 and 10-6 conferred significantly inferior PFS. Overall, this study defines MRD-negativity as the most relevant clinical endpoint for both standard- and high-risk transplant-eligible MM patients.
Paiva: Sanofi: Consultancy, Honoraria, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria; Merck: Honoraria; Novartis: Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; EngMab: Research Funding. Oriol: Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia; Celgene: Speakers Bureau. de la Rubia: Janssen: Other: Honoraria; Amgen: Other: Honoraria; Celgene: Other: Honoraria. Rosinol: Celgene: Honoraria; Janssen: Honoraria. Mateos: Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Lahuerta: Amgen: Honoraria; Celgene: Honoraria; Janssen: Honoraria. San Miguel: Roche: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal